New material enables chemical production using sunlight and water
An international team of researchers from the Czech Republic, Germany and China has used nanotechnology and atomic engineering to develop a groundbreaking material that can transform a wide range of organic compounds into desired products under ambient conditions, powered solely by solar energy and using water as a proton source. The material’s development marks a significant step towards finding alternatives for the environmentally and economically demanding hydrogenation reactions widely used in organic chemistry and pharmaceutical and agrochemical production. The study, which includes contributions from scientists at the Czech Advanced Technology and Research Institute (CATRIN) at Palacký University, was published in the prestigious journal Advanced Materials.
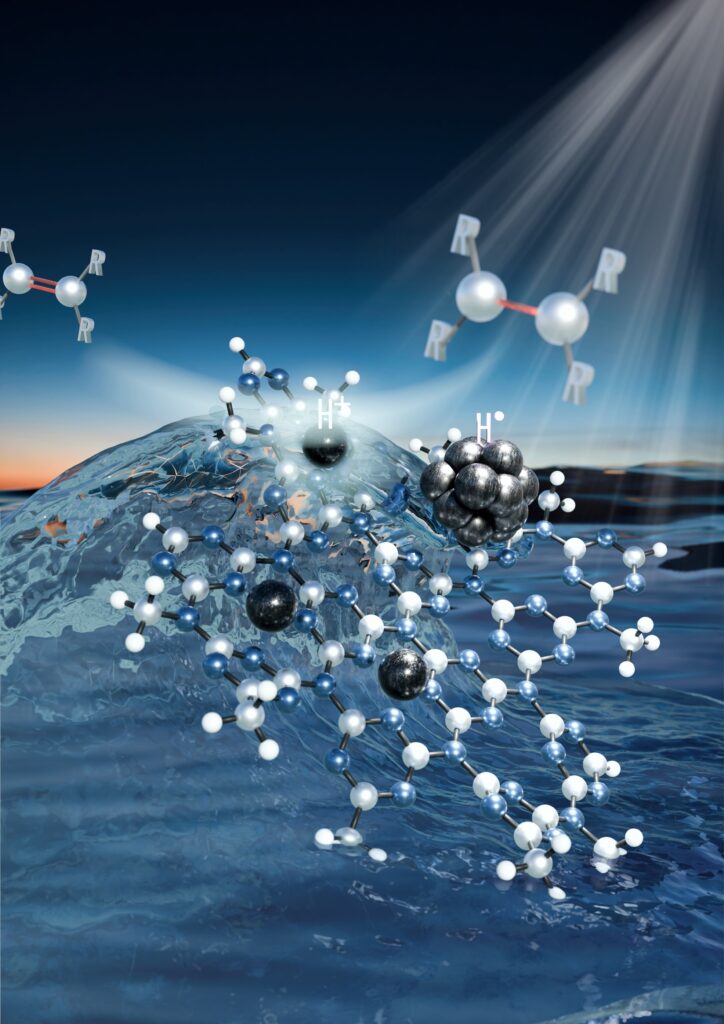
Hydrogenation reactions are integral to hundreds of chemical manufacturing processes across agrochemistry, pharmaceuticals, industrial chemistry and many other sectors, with a combined market worth tens of billions of dollars. However, many photocatalysts currently in use are unable to achieve the yields required for industrial-scale applications and suffer from limited selectivity, meaning they struggle to steer chemical reactions towards the desired products. Moreover, they often require the assistance of additional agents, such as water activation using magnesium, and their applicability is restricted to a narrow range of organic reactions. Finding new solutions that, unlike existing ones, can operate under low temperatures and pressures and without the use of gaseous hydrogen, represents a major scientific and industrial challenge. One promising approach involves using water as a proton source in combination with suitable photocatalysts that enable efficient transformation powered by solar energy.
“In developing a new type of photocatalyst, we combined expertise in nanotechnology and atomic engineering,” said corresponding author Radek Zbořil. “Together with our international collaborators, we designed and synthesized a material consisting of palladium nanoparticles anchored within a two-dimensional carbon nitride matrix. Isolated palladium atoms in various oxidation states were incorporated in the vicinity of these nanoparticles. Thanks to the synergistic effect of the components, the new material managed to convert a broad range of organic compounds into desired products with exceptional yields and selectivities, paving the way for industrial applications.”
During the research, the team observed that the reaction yields increased dramatically when isolated palladium atoms in varying oxidation states were located near the nanoparticles. “We therefore intentionally designed a composite system in which isolated palladium atoms attracted photogenerated holes to oxidize water, while nanoparticles facilitated the transfer of hydrogen to unsaturated bonds in organic molecules. This is a unique concept that could revolutionize catalytic processes in organic chemistry,” explained Giorgio Zoppellaro, who played a key role in elucidating the material’s mechanism of action.
The research teams from CATRIN and VSB-TUO, in collaboration with colleagues from Germany’s Leibniz Institute for Catalysis, have been investigating hydrogenation reactions for years. They have already published several pioneering studies focused on new technologies for synthesizing amine compounds (e.g. Chandrashekhar et al. Nat. Catal. 2022; Cheruvathoor Poulose et al. Nat. Nanotechnol. 2023).

