Computational chemists from CATRIN throw light on molecular photoswitching
Collaboration of CATRIN scientists with world-renowned teams including the one of the Nobel Prize Winner Prof. B. L. Feringa bears fruits. Computational chemists from CATRIN once again contributed to the elucidation of photochromic behavior of an important class of molecular photoswitches. This time, they focused on azonium ions which photoisomerize with red light under physiological conditions. This property makes them attractive as molecular tools for the photocontrol of physiological processes and is the topic of a paper recently published in Journal of American Chemical Society.
A rapidly growing interest in the development of molecular photoswitches stems from the human dream to precisely control properties and functions of materials at the atomistic level analogously to daily-life ON-OFF devices, which can be achieved by light. Compared to other external stimuli, the activation of photo-responsive materials by light is non-invasive and highly localized, which has found countless applications in biomedicine and pharmacology (e.g., the photochemical control of drugs, drug delivery, bioimaging) as well as in nanotechnology (e.g., healable and recyclable materials, reversible adhesives, data storage, and energy storage).
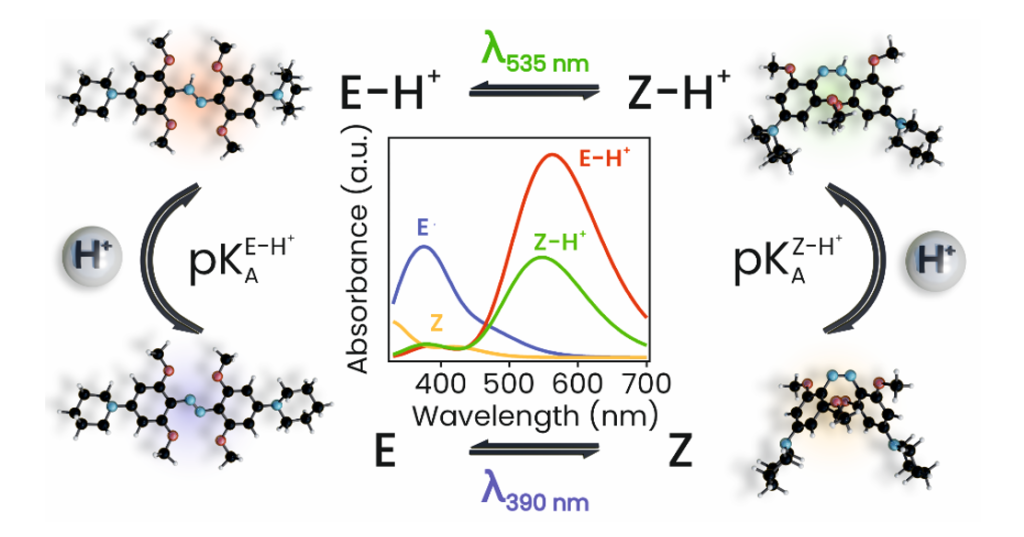
For biomedical applications, photoswitches that operate in the red/near-IR spectral region are of particular interest. Red light is highly penetrating in biological tissues and has minimal adverse effects. However, very few molecular photoswitches can isomerize in aqueous conditions with red light in a reversible manner without degradation. Azoniums appear to be very promising candidates, however, they are typically formed at low pH (<3), and their thermal isomerization occurs on the microsecond time scale, which makes them unsuitable for biological applications. A notable exception is the azonium ion formed by protonation of tetra-ortho-methoxy-substituted di-aminoazobenzene, which is stable at pH 7, photoisomerizes with red light, and thermally relaxes on the time scale of seconds. These properties make it ideal for photopharmacology applications such as in vivo enzyme inhibition and targeting receptor signalling.
“For the rational application of azoniums as well as for guiding the design of their derivatives with improved properties, a mechanistic understanding of the photoisomerization process and subsequent thermal relaxation is crucial. Using a combination of ultrafast transient absorption measurements and quantum chemical calculations, we fully explained reaction mechanisms of both photo-actinic and thermal steps as well as their pH dependence. Based on these findings, we are now exploring new azonium derivatives for optoacustic imaging applications, but let us keep this for the next story,” says Miroslav Medveď, the first co-author of the JACS paper.

